Johnson & Johnson's Coronavirus Vaccine Trials Paused After Participant Gets 'Unexplained Illness'
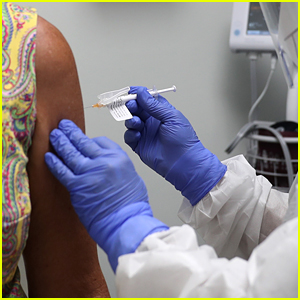
Johnson & Johnson has announced that it has paused coronavirus vaccine trials after a participant developed an “unexplained illness.”
STAT reported that the company was running a 60,000-patient clinical trial for a COVID-19 vaccine, but they sent out a document stating a “pausing rule.”
Johnson & Johnson confirmed to the outlet that the trials are on pause because of “an unexplained illness in a study participant.”
“We must respect this participant’s privacy. We’re also learning more about this participant’s illness, and it’s important to have all the facts before we share additional information,” the company said in a statement.
The company also noted that the vaccine study is not on a clinical hold, which would be a more serious matter. Clinical trial pauses are reportedly not uncommon.
On September 23, President Trump reacted to the news that J&J was in the final stage of clinical trials on the vaccine and he urged the FDA to move quickly on approving the vaccine.
“Big news. Numerous great companies are seeing fantastic results. @FDA must move quickly!” Trump said at the time.
Big news. Numerous great companies are seeing fantastic results. @FDA must move quickly! https://t.co/2pDrmRPOxc
— Donald J. Trump (@realDonaldTrump) September 23, 2020
NOTE: The photos in this post are stock images and aren’t connected to the Johnson & Johnson vaccine trial.










 Older
Older





